Researchers have grown mice with brains made partly of rat cells, allowing them to smell the world through another animal’s neurological hardware.
It’s the first time an animal has used another’s senses to detect and respond to its surroundings, demonstrating how adaptable the brain can be in accepting different types of brain cells.
The US team says the findings could help identify similarities in how brains develop, change, and repair across different species, while potentially informing research into human-machine interfaces or stem cell transplantation.
“Right now, researchers are transplanting stem cells and neurons into people with Parkinson’s and epilepsy,” says geneticist Kristin Baldwin from The Scripps Research Institute in the US.
“With hybrid brain models, we can start to get some answers and at a faster pace than a clinical trial.”
Researchers have previously made hybrid brains by transferring neurons or small brain-like structures called organoids from one species into another, such as rat tissues grown in mice. However, these methods had limitations because the added cells didn’t always connect properly with the existing brain.
Baldwin and colleagues wanted to know if they could make synthetic neural circuits from two different species work together in a living brain.
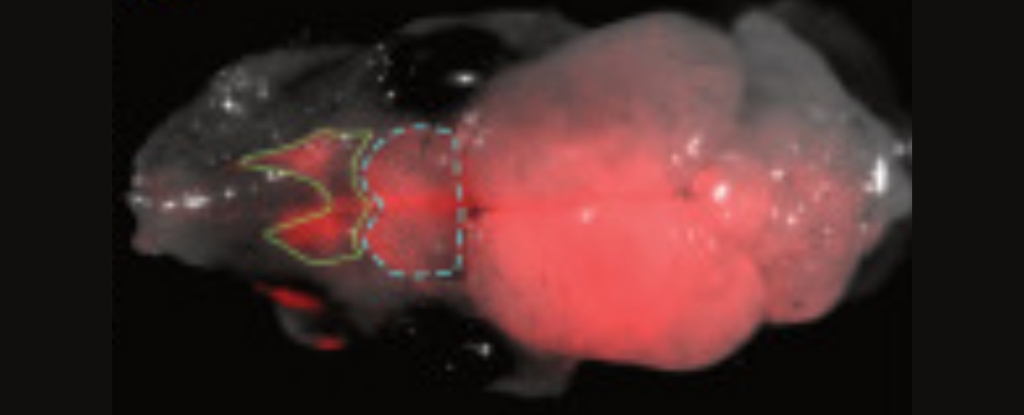
Using a technique called blastocyst complementation, they introduced rat stem cells into mouse embryos shortly after fertilization, which allowed the rat and mouse cells to grow and integrate together naturally. It’s the first time this specific process has successfully resulted in hybrid brains made using cells derived from two different species.
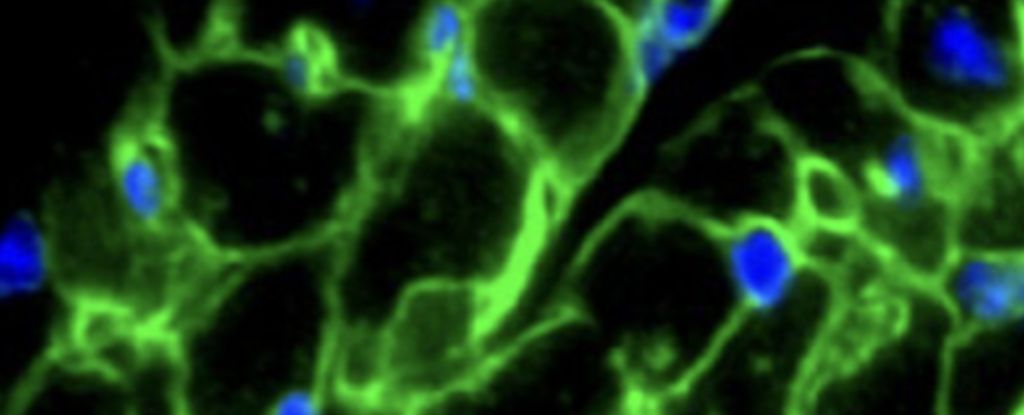
Surprisingly, the mouse brain environment altered the timing of rat neuron development and supported synaptic connections between rat and mouse neurons, even though rats and mice have evolved separately for millions of years and have differences in brain size.
“You could see rat cells throughout almost the entire mouse brain, which was fairly surprising to us,” Baldwin explains. “It tells us that there are few barriers to insertion, suggesting that many kinds of mouse neurons can be replaced by a similar rat neuron.”
When mouse neurons involved in smell were turned off or killed, rat neurons took over and helped the brain process smells and find food.
“We hid a cookie in each mouse cage, and we were very surprised to see that they could find it with the rat neurons,” Baldwin says.
Curiously, retaining dead and silent neurons still appeared to be having an effect on the animals’ behavior. Mice with inactivated olfactory neurons had a harder time finding concealed cookies than mice whose olfactory neurons were engineered to vanish altogether during their development stage.
“If you want a functional replacement, you may need to empty out dysfunctional neurons that are just sitting there,” Baldwin suggests, “which could be the case in some neurodegenerative diseases and also in some neurodevelopmental disorders like autism and schizophrenia.”
Although the results are promising, we’ll need more research to understand the implications of interspecies neural integration and leverage its potential.
One issue with the new hybrid brain system is that the rat cells are randomly dispersed, making it difficult to apply the method to other brain systems.
Baldwin’s team is working on directing these cells to become a specific type, which could improve experimental precision. If successful, this method could even result in hybrid brains with primate neurons.
“This would help us get even closer to understanding human disease,” Baldwin says.
The research has been published in Cell.