The inner workings of our brain’s ‘sewage system’ are finally coming to light after years of speculation.
Deep within the crevices of five human brains, scientists have, for the very first time, imaged the dynamic intricacies of a underlying plumbing network – previously seen in mice and only hypothesized in our own species.
The findings support the existence of a glymphatic system, which scientists first identified in the brains of mice in 2012.
This system carries cerebrospinal fluid (CSF), which bathes the outside of the brain, into the interior, delivering nutrients and removing waste products – such as proteins that form clumps in Alzheimer’s disease – from brain tissue.
Since 2012, several pioneering studies have shown the glymphatic system also exists in human brains, but without seeing the fluid moving from the outside of the brain into the space between neurons, that idea has remained highly controversial.
“I was always skeptical about it myself, and there are still a lot of skeptics out there who still don’t believe it,” says neurologist Juan Piantino from OHSU.
“That’s what makes this finding so remarkable.”
Piantino and his colleagues at OHSU are the first to image the colorless liquid as it flows into the tissue of a living human brain, and the results support previous imaging studies that took only a brief snapshot.
The research was made possible with the consent of five adults undergoing brain surgery, who needed their CSF to be diverted for the procedure.
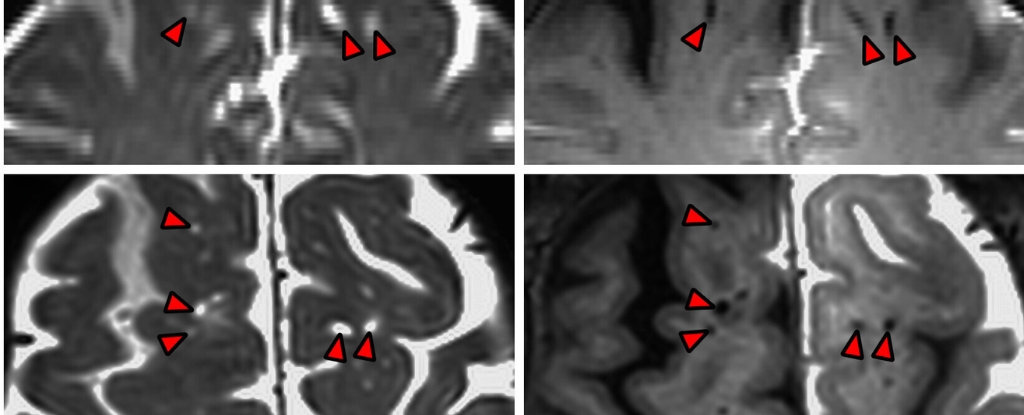
Before the fluid was replaced, scientists marked it with a dark contrast tracer. Later, a special type of magnetic resonance imaging mapped where the fluid had gone in the brains of participants.
The findings suggest the human brain doesn’t randomly absorb CSF, like a sponge would. Instead, the fluid penetrates deeper into neurological tissue by following in the tracks of blood vessels.
These clearly defined channels of CSF are actually wrapped around the outside of blood vessels. The borders of these ‘perivascular spaces’ are formed from brain cells, which stick their ‘endfeet’ together to create a permeable barrier.
Some scientists hypothesize it is this membrane that allows CSF to mix with the fluid that cushions and supports our brain cells, delivering nutrients and removing waste.
OHSU neurosurgeon Erin Yamamoto says that in the MRI images from their study, “you can actually see dark perivascular spaces in the brain turn bright” over time, as the contrast tracer flows deeper.
“It was quite similar to the imaging… in mice,” she adds.
These CSF channels have previously been imaged in human brains, but because researchers at OHSU took MRI scans 12 hours, 24 hours, and 48 hours after surgery, they were able to track the fluid’s dynamics in a way that hadn’t been seen before.
The findings show CSF channels are not stagnant, fluid-filled structures, but “functional conduits”, facilitating “the distribution of CSF into the brain”.
“People thought these perivascular spaces were important, but it had never been proved,” Piantino says.
“Now it has.”
The study was published in PNAS.