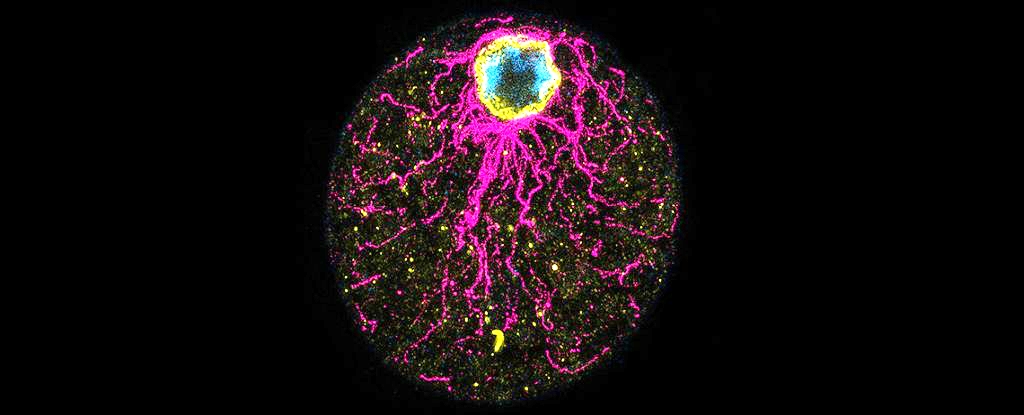
Life on Earth may have developed the ability to form embryos even before it grew the very first animals.
A single-celled organism that lives burrowed in the muck beneath shallow seas bears a startling resemblance to animal embryos as it reproduces, says a team of scientists led by biochemist Marine Olivetta of the University of Geneva. The way it divides itself resembles the process of embryonic cell division.
The organism in question is an Ichthyosporean microbe called Chromosphaera perkinsii, and since it has been around for over a billion years, long before the first animals emerged, its existence suggests that life developed the programming for eggs before the eggs themselves.
“Although C. perkinsii is a unicellular species,” explains biochemist Omaya Dudin of the Swiss Federal Institute of Technology, “this behavior shows that multicellular coordination and differentiation processes are already present in the species, well before the first animals appeared on Earth.”
Multicellular animal organisms all start their lives the same way. Two zygotes merge and fuse, kicking off the process of cell division and growth. The initial stages of cell division are called cleavage, during which a lot of cell division takes place rapidly without growth, a process known as palintomy.
The resulting product of this process is a clump of cells, hollow in the middle, like a raspberry. This is called the blastula.
This process in animals is also observed in unicellular organisms as a means of reproduction. The organism divides itself into multiple daughter cells that split off and become independent. And, in fact, scientists have drawn parallels between the two before. A paper published earlier this year proposed that Ichthyosporeans may be an excellent model for understanding the origins of animals.
That’s because Ichthyosporeans represent a class of unicellular organisms that diverged more than a billion years ago from the lineage that would go on to produce animals. They are not animals, but they are closely related, and any similarities between animals and Ichthyosporeans could have been inherited from a common ancestor before the lineages diverged.
Earlier this year, a team of scientists that included Olivetti published a paper describing palintomic reproduction in C. perkinsii in a manner similar to animal mitosis.
Dudin and his colleagues conducted a study of C. perkinsii – one of the very few Ichthyosporeans that isn’t a parasite – comparing it to several other members of the class, to see if any further similarities could be identified between palintomic reproduction and animal embryonic cleavage.
They discovered that, following palintomy, C. perkinsii forms a cluster of cells just like a blastula. And there are at least two distinct cell types within that colony. The conglomeration of cells then hangs out in that blastula-like colony for a significant proportion of its life cycle, before the cells eventually scatter and wriggle off to do their own thing.
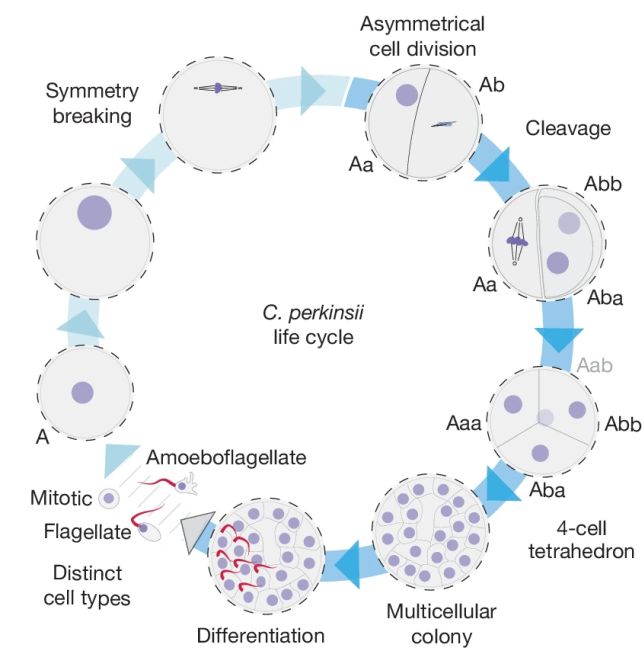
It’s startlingly similar to how an animal embryo develops. This suggests that the development could be ancestral between animals and Ichthyosporea, and that the genetic programming for embryonic development was in place before embryos came into existence.
But nature is also very good at replicating things that work. Sometimes traits or processes emerge independently in very different organisms, a process known as convergent evolution. The development and colony clustering of C. perkinsii seems unusual; it hasn’t been observed in other Ichthyosporeans.
In fact, apart from some scattered partial observations, no other animal relative develops similarly to the animal embryo or C. perkinsii. This suggests convergent evolution might be the answer; but we can’t rule out a common ancestor, either.
Either way, we have fascinating things to learn. On the one hand, C. perkinsii could reveal new insights into the evolutionary origins of all animals. On the other, it suggests the genetic toolkit available to early life was much more versatile than we thought.
“Future research will be essential to elucidate how spatial cell differentiation is established in C. perkinsii,” the researchers write in their paper. “Nevertheless, our study indicates that C. perkinsii represents a transitional form between temporal and spatial cell differentiation, providing insights into the evolutionary mechanisms that led to emergence of animal multicellularity.”
The research has been published in Nature.