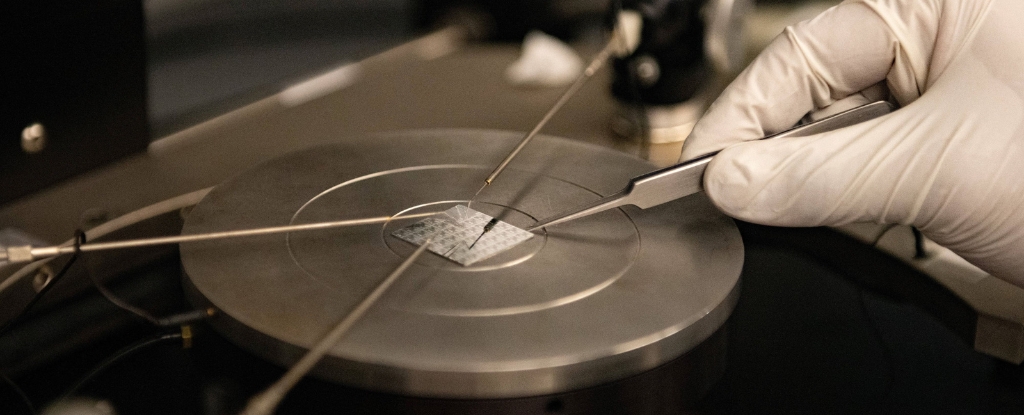
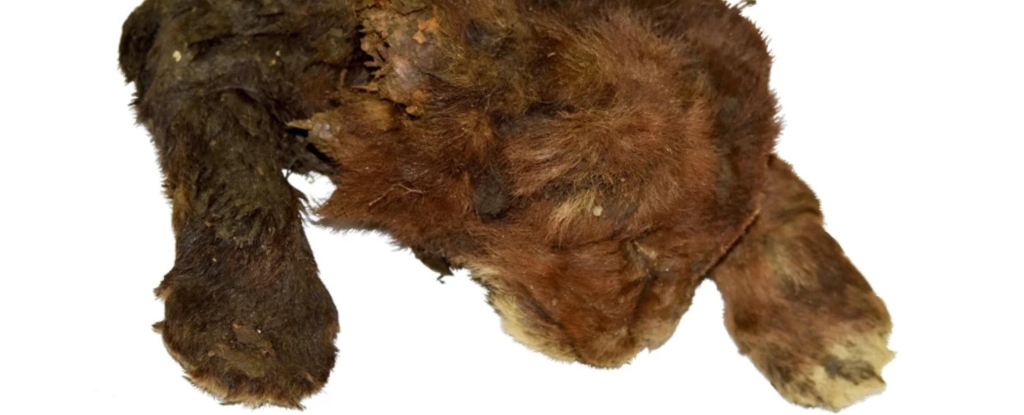
The deadliest pandemic in recorded history may still continue to plague human oral health centuries later, potentially contributing to some modern cases of gum disease.
That’s still very much a hypothesis, but if the correlations can stand up to future research, it could reveal an unexpected, long-lasting consequence of contagious respiratory diseases.
The microbiome that lives in the human mouth, nose, and pharynx is the second-largest microbial community in our bodies after the gut. Now, one of the first studies to track its evolution through history has found a pivotal transition that coincides with the Black Death of the Late Middle Ages.
The research, which was led by scientists at Pennsylvania State University, is based on the genetic analysis of ancient dental samples from 235 individuals living in Great Britain between about 2200 BCE and 1853 CE.
More than half of these individuals lived in London, where waves of bubonic plague over several centuries claimed the lives of many tens of thousands of individuals.
This city alone lost between 30 and 50 percent of its population in just three years following its emergence in the 14th century, “changing the population structure and ways of life in the city substantially,” say the international team of scientists.
Across all the ancient British teeth analyzed, the team identified 954 microbial species that broadly fall into two distinct communities.
These communities seem to have shifted in composition after the arrival of the second plague pandemic in London in 1348.
A microbial community dominated by the genus Methanobrevibacter emerged about 2,200 years ago, although it is now largely extinct in modern Industrialized people, after disappearing around 1853
The more modern microbial community, meanwhile, showed lower bacterial diversity and was largely dominated by the genus Streptococcus – a community that tends to support bacteria linked to periodontal disease.
This suggests that the modern origins of gum disease “may in fact originate from the Streptococcus-associated communities,” the authors write.
The bubonic plague is not responsible for all these changes, but according to statistical analyses, time-related factors that include the arrival of the Black Death could explain nearly 11 percent of the historical shift in oral microbiomes in London.
“While this finding needs further examination, temporal shifts in oral microbiome composition coinciding with the Second Plague Pandemic in London could be the result of disease selection and susceptibility during the pandemic,” write the researchers. They also suggest the advancements in public hygiene, diet and culture that followed the plague could have contributed to changes in the microbiome of the human mouth.
“We know that survivors of the Second Plague Pandemic earned higher incomes and could afford higher-calorie foods,” explains anthropologist Laura Weyrich from Penn State.
“It’s possible that the pandemic triggered changes in people’s diets that, in turn, influenced the composition of their oral microbiomes.”
After the Black Death, for instance, folks in London ate more more expensive foods, such as fish, meat, and wheat bread.
The pandemic may have also shifted the genetics of the city population in a way that indirectly impacted the oral microbiome.
Recent DNA analysis from victims of the Black Death suggests that this deadly pandemic triggered genetic changes in the human population that persist to this day.
People in the 14th century who possessed the genetic variation, ERAP2, for instance, were roughly 40 to 50 percent more likely to survive if they contracted the plague-causing bacterium Yersinia pestis.
Today, identical copies of the ERAP2 gene have been found to possibly lower the risk of respiratory infections, like COVID-19, while at the same time, increasing the risk of autoimmune diseases, like Crohn’s, type 1 diabetes, and rheumatoid arthritis.
Changes to the population’s genetic makeup following the plague may have, therefore, increased the risk of diabetes. Then, the increased risk of diabetes may have increased the risk of gum disease in turn.
The team doesn’t yet know why the Methanobrevibacter-associated community disappeared in Britain, or if the Streptococcus-dominated community is, in fact, a leading cause of periodontitis. But Weyrich and her colleagues did find that, at least in London, periodontal disease was significantly linked to oral microbiome composition.
In London, Weyrich and her colleagues found periodontal disease was significantly linked to oral microbiome composition.
The authors behind the study cannot prove cause and effect with their results, but the indirect associations that seem to connect respiratory diseases, immune issues, and oral health enough to suggest it should be investigated further.
“Uncovering the origins of these microbial communities may help in understanding and managing these diseases,” says Weyrich.
The study was published in Nature Microbiology.