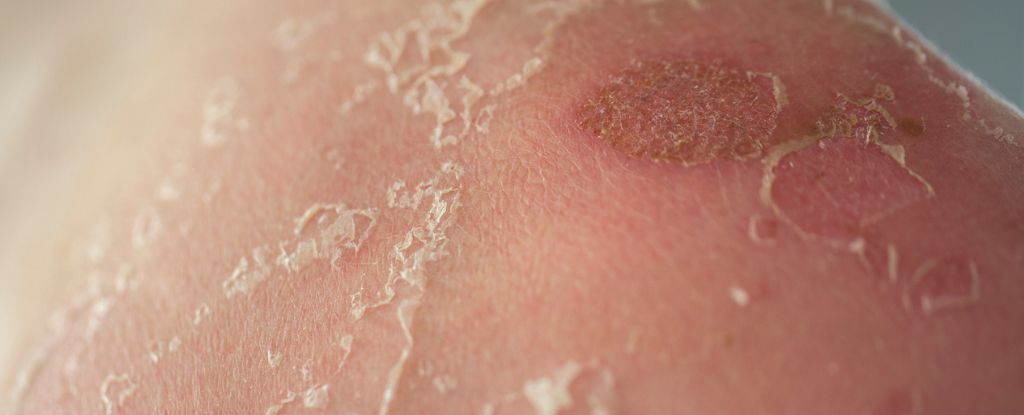
A drug already used to alleviate some of the painful symptoms of rheumatoid arthritis might also be effective at stopping it from developing to begin with – potentially saving millions of people from this debilitating condition.
The drug in question is abatacept, and it’s been part of a trial set up to test its effectiveness and safety. This involved 213 patients at high risk of developing rheumatoid arthritis in the future, based on early symptoms such as joint pain.
For the phase 2b clinical trial, 110 participants were randomly selected to be given abatacept, and 103 participants were given a placebo for a year, with the volunteers’ progress then followed up for a further 12 months.
The results were significant: after the first year, 29 percent of the placebo group had developed rheumatoid arthritis, compared to only 6 percent of the abatacept group. After the second year, those stats rose to 37 percent of the placebo group and 25 percent of the abatacept group.
“This is the largest rheumatoid arthritis prevention trial to date and the first to show that a therapy licensed for use in treating established rheumatoid arthritis is also effective in preventing the onset of disease in people at risk,” says Andrew Cope, a rheumatologist from King’s College London in the UK who led the study.
“These initial results could be good news for people at risk of arthritis as we show that the drug not only prevents disease onset during the treatment phase but can also ease symptoms such as pain and fatigue.”
Rheumatoid arthritis is brought on by the body’s immune system attacking its own tissues, and abatacept works by dampening down the response of T cells, which play a key role in the immune system.
While the results are promising and show some evidence of a lasting effect, further study will be required. The trial only covered two years, so it’s possible that abatacept only delays arthritis rather than prevents it.
“The data indicates that abatacept treatment beyond 12 months might be required to sustain efficacy over time,” write the researchers in their published paper. “Intermittent administration at intervals remains to be assessed.”
The abatacept group experienced less pain and inflammation, and scored higher in quality of life measurements. However, it’s also worth noting that the drug can come with mild side effects, including nausea and diarrhea.
Rheumatoid arthritis is a chronic disease that can be intensely painful for those who suffer from it – and while we’re still at the early stages in terms of this research, the hope is that abatacept and drugs like it could eventually prevent more suffering.
“There are currently no drugs available that prevent this potentially crippling disease,” says Cope.
“Our next steps are to understand people at risk in more detail so that we can be absolutely sure that those at highest risk of developing rheumatoid arthritis receive the drug.”
The research has been published in The Lancet.