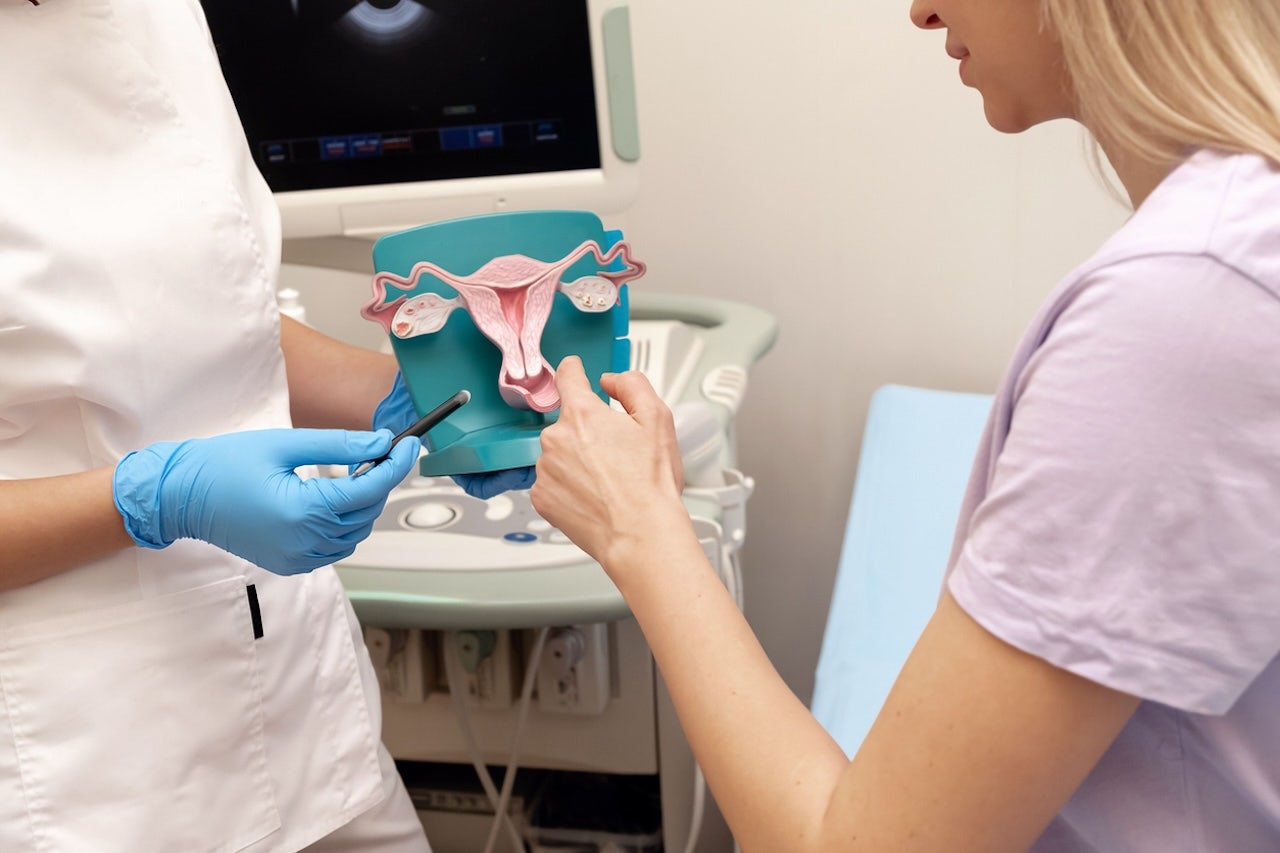
The U.S. Food and Drug Administration (FDA) has approved a new drug for certain adult patients with endometrial cancer.
Jemperli (dostarlimab-gxly) — made by British pharmaceutical company GSK — is intended for people with primary advanced or recurrent forms of the cancer, according to the FDA’s Aug. 1 announcement.
It is an immunotherapy-based drug, which means it leverages the body’s immune system to attack cancer cells.
SOME TAMPON PRODUCTS FOUND TO CONTAIN TOXIC METALS IN FIRST-TIME STUDY: ‘HARMFUL INGREDIENTS’
In clinical trials, Jemperli showed an improved progression-free survival and overall survival for all patients, according to Dr. Brian Slomovitz, director of gynecologic oncology and co-chair of the Cancer Research Committee at Mount Sinai Medical Center in Florida.
“This class of drugs has been used in the second-line setting,” Slomovitz said — meaning they were only used if a first-line (primary) treatment was not effective or had intolerable side effects.
The FDA has approved a new drug for certain adult patients with endometrial cancer. (iStock)
“Moving them to first-line [status] here will yield better outcomes.”
This is the third FDA approval this year for immunotherapy in endometrial cancer, the doctor noted.
THE 9 MOST COMMON QUESTIONS WOMEN OVER 40 ASK THEIR DOCTORS, ACCORDING TO A MENOPAUSE EXPERT
“This is great news for our patients,” Slomovitz told Fox News Digital.
“Endometrial cancer has become the leading cause of death over all other gynecologic cancers, including ovarian cancer.”
Jemperli (dostarlimab-gxly) — made by British pharmaceutical company GSK — is intended for people who have primary advanced or recurrent forms of the cancer. (iStock)
Before the immunotherapy options became available, the only treatment for endometrial cancer was chemotherapy alone, Slomovitz said.
“These results are game-changing — it is unprecedented to have three approvals in such a short period of time.”
The most common side effects of all immunotherapy drugs are gastrointestinal, endocrine and dermatologic toxicities, the doctor noted.
“These results are game-changing — it is unprecedented to have three approvals in such a short period of time.”
“Patients should understand that adding immunotherapy to traditional chemotherapy has better results and the toxicity profile is manageable,” he added.
For doctors, Slomovitz emphasized the need to adjust quickly and make the newly approved drug a first-line treatment option for their patients.

GlaxoSmithKline (GSK) is the U.K.-based pharmaceutical company that makes Jemperli. (REUTERS/Dado Ruvic/Illustration/File Photo)
In June, the FDA approved Merck’s Keytruda (pembrolizumab) to be used in combination with chemotherapy for adult patients with primary advanced or recurrent endometrial cancer.
“The Merck trial (pembrolizumab) showed a significant improvement in progression-free survival for all patients,” Slomovitz said.
CLICK HERE TO GET THE FOX NEWS APP
In that same month, AstraZeneca’s Imfinzi (durvalumab) was approved for patients with advanced or recurrent disease who have a certain biomarker.
The AstraZeneca trial also showed improved progression-free survival for all patients.
All trials for the new cancer drugs were run through the GOG Foundation, which is the largest cooperative group for all gynecologic oncology research, Slomovitz noted.

“The addition of immunotherapy to chemotherapy provided improvement in survival outcomes without a negative impact on quality of life,” an oncologist said. (iStock)
In the trials, the use of immunotherapy drugs in combination with chemotherapy reduced the risk of disease progression by approximately 70% in patients whose tumors had certain biomarkers.
In other patients without that biomarker, there was still a “clinically and statistically significant improvement in time to progression, which is meaningful for our patients and has now led to FDA approvals in both biomarker settings,” Kathleen N. Moore, M.D., co-director of the Stephenson Cancer Center at the University of Oklahoma Health Sciences Center, told Fox News Digital.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
“These studies have transformed outcomes for patients with endometrial cancer and are poised to change the treatment landscape for the better into the future,” she added.
Shannon N. Westin, medical director of the Gynecologic Oncology Center at the University of Texas MD Anderson Cancer Center, said she is “thrilled” to have multiple new options for patients with advanced and recurrent endometrial cancer after years with no new therapies.

This is the third FDA approval this year for immunotherapy in endometrial cancer, the doctor noted. “This is great news for our patients,” an oncologist told Fox News Digital. (iStock)
“We no longer have to treat everyone in the same cookie-cutter fashion, and can instead employ precision medicine to improve survival for these survivors.”
For more Health articles, visit www.foxnews/health
“The addition of immunotherapy to chemotherapy provided improvement in survival outcomes without a negative impact on quality of life,” she went on.
“This is the ideal scenario.”
Fox News Digital reached out to GSK for comment.