A gene that causes skull malformation when mutated has just been linked to schizophrenia, a condition known for causing unsettling hallucinations and disordered thinking.
“This would be extraordinary because it all started with a bone,’ says St. Jude Children’s Research Hospital neurobiologist Stanislav Zakharenko.
Zakharenko supervised the team that revealed the connection. If it holds true it shows how brain disorders can sometimes arise from a disruption in the communications from other tissues rather than out of the brain tissue itself.
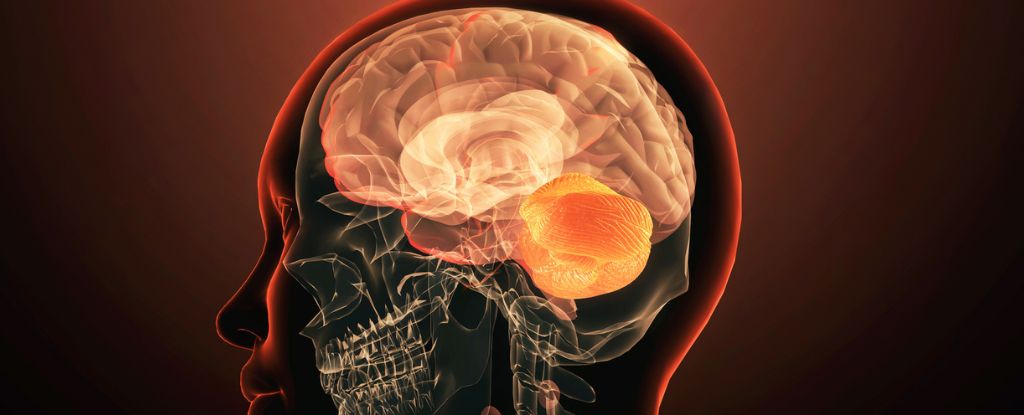
Led by Zakharenko’s colleague Tae-Yeon Eom, the researchers found that in mouse models deletion of one copy of the Tbx1 gene disrupts skull bone formation during development, leading to a malformed pocket in the brain’s casing. Without an appropriately shaped skull, lobes of the cerebellum that usually grow within are 70 percent smaller than normal.
“What is interesting about Tbx1 is that it is not very well expressed in the brain, especially adolescent or adult brain,” explains Zakharenko. “Rather, it’s expressed in the surrounding tissues, namely bone, cartilage, and vasculature tissues. It is very unlikely that Tbx1 directly affects the brain at all.”
Yet mice without Tbx1 show motor-learning difficulties similar to humans with a mutation known to be associated with schizophrenia.
So the researchers used MRI scans to investigate the brains of 80 human patients with 22q deletion syndrome, who have a missing section of chromosome 22 containing about 25 different genes. Up to 30 percent of people with this syndrome are diagnosed with schizophrenia at some point in their life, while just 1 percent of those without the deletion receive a diagnosis.
Previous research on 22q deletion syndrome revealed disruptions in the routes taken by auditory information as it travels from the thalamus to an area of the cortex where the sound stimuli are interpreted.
“We think that reducing the flow of information between these two brain structures that play a central role in processing auditory information sets the stage for stress or other factors to come along and trigger the ‘voices’ that are the most common psychotic symptom of schizophrenia,” explained Zakharenko in 2014.
Sure enough, MRI scans revealed the human patients also have smaller flocculus and paraflocculus lobes, just as the team had observed in the mice. Tbx1 appears to be one of the genes impacted by the deletion.
As the paraflocculus is also connected to the auditory cortex the team suspects the Tbx1 mutation may be contributing. Previous research has also shown Tbx1 is involved in our startle response to noise, as well as inner ear defects and other brain conditions too.
“In humans without [22q], rare variants of Tbx1 have been associated with autism-spectrum disorder and schizophrenia,” Eom and team write in their paper.
“Although thalamocortical disruption occurs late in development, which is consistent with the onset of schizophrenia symptoms, it stays and doesn’t go away. However, hallucinations are transient in nature – they come and go,” says Zakharenko. “It seemed that this was just one of the hits that triggered symptoms. The question is: What is the other hit?”
Further research is required to directly connect all these puzzle pieces together.
“In my mind, it’s like a stepping stone. We hope to follow this chain of events from the malformed skull to the underdeveloped flocculus and paraflocculus to the auditory cortex dysfunction,” Zakharenko concludes.
As with many neurological conditions, the exact causes of schizophrenia are still largely unknown.
Current treatments only work for some patients, leaving others struggling with the debilitating symptoms. This suggests there may be multiple factors responsible including genetic, environmental, and biological changes.
This research was published in Nature Communications.