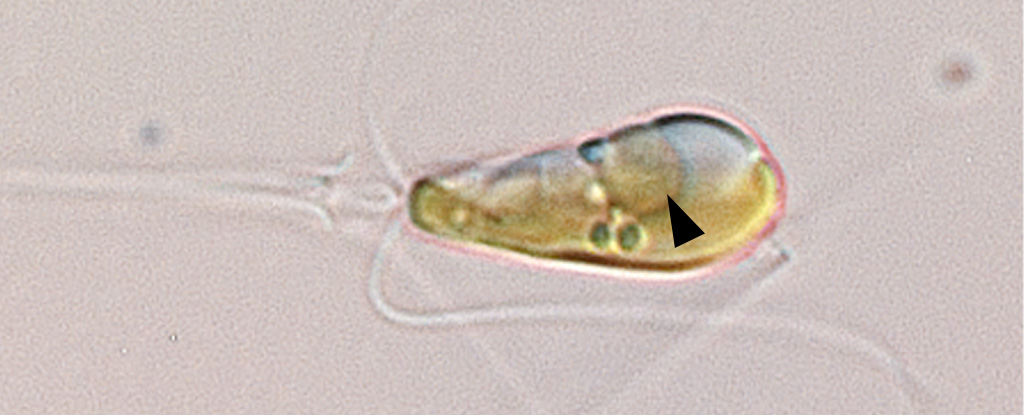
Scientists have discovered a species of marine algae that contains an organelle capable of harvesting nitrogen from the atmosphere – an ability previously thought to be the exclusive domain of certain symbiotic bacteria.
The international team of researchers behind the discovery believe the algae’s talent originated from an endosymbiotic relationship, where a nitrogen-fixing cyanobacterium was engulfed by an ancestral marine algal cell. Then, in an extreme case of codependence, the bacteria gave up on being its own organism altogether, discarding many of its genes and becoming reliant on the unicellular algae for support.
It’s far from the only example of a microbial marriage resulting in a cellular organ forming. Superstars of biochemistry like chloroplasts (the tiny factories that turn sunlight into energy, without which most life on Earth would be impossible) and mitochondria (the powerhouse of the cell, producing useable forms of energy) also started as a microbial share-house arrangement.
Still, it’s not every day you come across an entirely new kind of organelle, which scientists have dubbed ‘the nitroplast’. In fact, this is only the fourth example of primary endosymbiosis ever recorded.
“It’s very rare that organelles arise from these types of things,” says University of California (UC) Santa Cruz biologist Tyler Coale, first author of one of two recent papers on the finding.
“The first time we think it happened, it gave rise to all complex life,” he says, referring to the origins of the mitochondria. “Everything more complicated than a bacterial cell owes its existence to that event.”
Then, a billion or so years ago, it happened again with the chloroplast, giving us plants.
Nitrogen-fixing bacteria are essential to the survival of plants all across the world. They are usually found living in root nodules, which you might’ve spotted if you’ve ever unearthed a pea or bean plant in your garden. Until now, it was assumed the only form this relationship could take was as a form of symbiosis.
The nitrogen-fixing structure was found in Braarudosphaera bigelowii and its relatives, marine algae found throughout the world’s oceans with a fossil record stretching back around 100 million years.
For decades, however, researchers struggled to culture the algae in the lab, so they couldn’t be sure if its nitrogen-fixing component, known as UCYN-A, was a symbiotic bacterium or had relinquished its independence to become an organelle.
Earlier this year, in March, UC Santa Cruz marine biologist Jonathan Zehr and other collaborators published a paper in Cell that showed UCYN-A indeed had the hallmarks of an organelle, including an increase in size correlated with the growth of its algal host, suggesting their metabolisms are intrinsically linked.
But it’s only once the embosymbiont starts “throwing away pieces of DNA”, Zehr explains, that symbiosis turns into, well, plain old biosis.
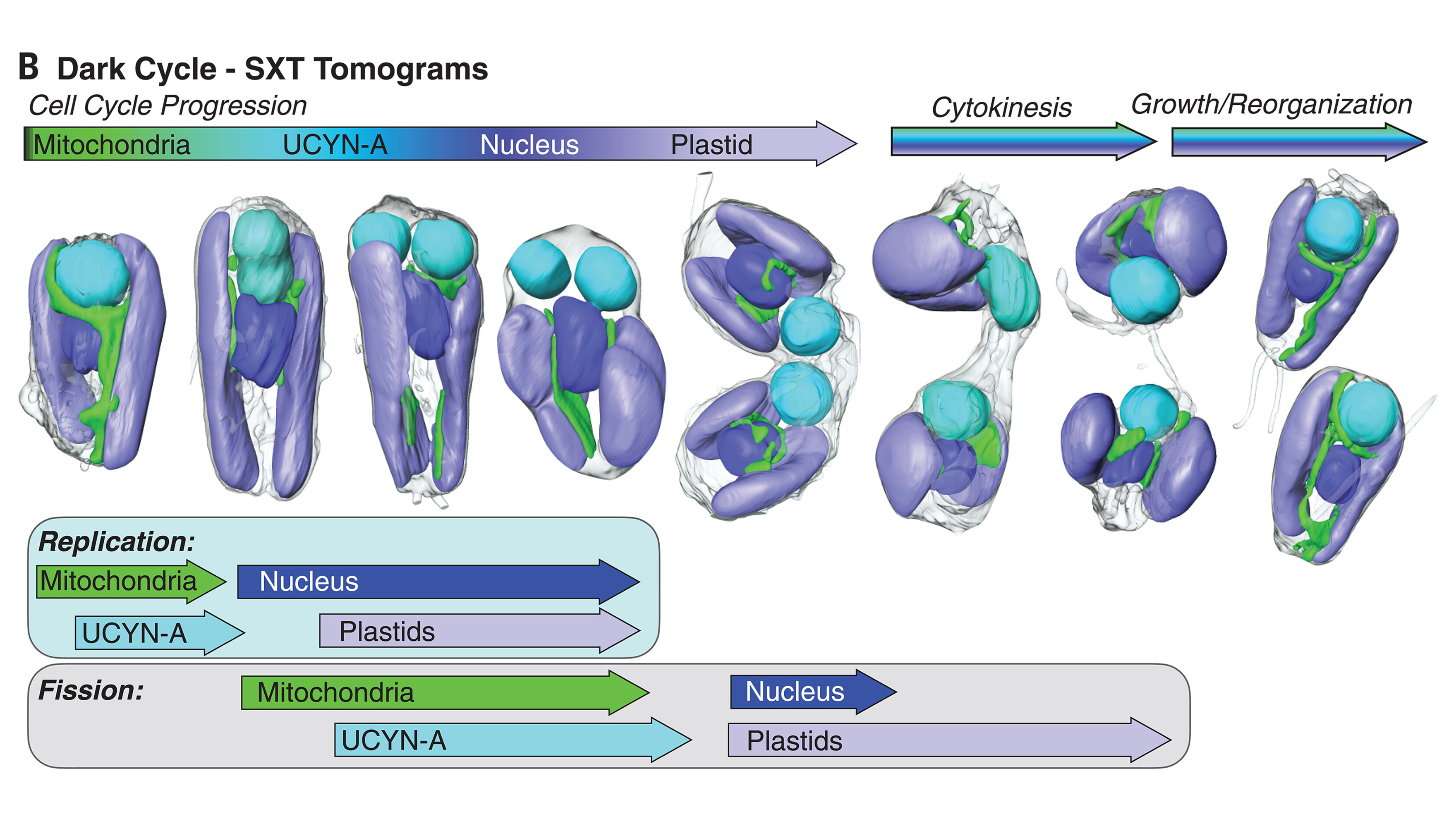
Since the symbiotic invader sits outside of the cell’s nucleus, its genetic material doesn’t get reshuffled during sexual reproduction along with the cell’s own DNA, preserving it through the generations. On the other hand, any DNA outside of the host’s nucleus is at greater risk of being damaged, increasing the odds the symbiotic guest can no longer function as an independent cell.
That’s what Coale, Zehr, and colleagues found in their subsequent study: UCYN-A’s genome had shrunk so much that it relied on importing proteins made by B. bigelowii.
“Their genomes get smaller and smaller, and they start depending on the mother cell for those gene products – or the protein itself – to be transported into the cell,” Zehr says.
These moments of two separate organisms merging disrupt linear notions of evolution, reminding us that even at the most basic levels, life is far more fluid – and interconnected – than we tend to realise.
But scientists think the discovery may have important implications for agriculture and marine science too. Different strains of UCYN-A have been found throughout the world’s oceans, from the tropics to the Arctic, so the nitroplast is likely a major contributor to the ocean’s protein production.