Aging might have advantages in wisdom, experience, and even evolution, but the gradual breakdown of biology that comes with growing old is rarely much fun for anybody.
A new study in mice suggests it may not always have to be this way.
“We demonstrated a way to delay aging and extend healthy lifespans in mice by manipulating an important part of the brain,” says Washington University developmental biologist Shin-ichiro Imai.
Our brains control a vast number of bodily functions, primarily by way of nervous impulses, which can manage communication networks based on the ebb and flow of hormones.
The infrastructure carrying these communication signals and their surroundings deteriorate as we age, causing increasing faults.
So our organs and tissues start to miss out on signals needed to maintain themselves.
Signaling chemicals that form parts of the pathway between our brains and fat tissues, specifically white adipose fat, have been associated with aging in mice previously, so Imai and colleagues took a closer look at the critical early steps in this communication network.
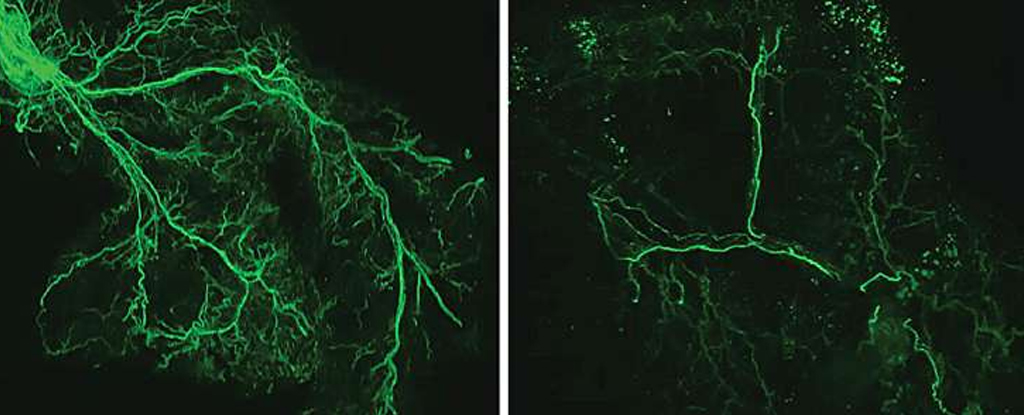
They allowed one group of rodents to age naturally while tweaking the neurons at the beginning of the brain to fat pathway so they remained active. These cells, DMHPpp1r17, are tucked away in our brain’s hypothalamus, an important conduit between our nervous system and the body’s hormone system.
Incredibly, the mice that received the neuron activation treatment lived 60 to 70 days longer than the control mice, who died within the typical laboratory mouse lifespan of about 1,000 days.
More importantly, the mice that underwent neural treatment had thicker, shinier coats and were more active during their old age, suggesting they were healthier for longer too.
Further investigation revealed parts of the mechanism at play.
When the DMHPpp1r17 neurons are on, they can activate our body’s fight or flight response – our sympathetic nervous system using the Ppp1r17 molecule. This promotes the use of our body’s white adipose stores that release a protein called eNAMPT, which in turn regulates hypothalamus neurons, completing the circuit.
“Ppp1r17 is also well conserved in various vertebrate species such as human, chimpanzee, monkey, rat, mouse, bovine, rabbit, chicken, xenopus, and zebra finch, suggesting that Ppp1r17 carries some essential functions throughout evolution,” the researchers explain in their paper.
This is a vital circuit for fueling our bodies, but the normal aging mice started producing less Ppp1r17, activating fewer fat stores.
With less activity, the nerves through our adipose tissues begin to degrade, meaning even less eNAMPT is produced, so even fewer hypothalamus neurons are activated, creating a self-propagating system of deterioration.
There are still many details to hash out, including if eNAMPT works directly on the hypothalamic neurons or if there are more intermediary steps. The team is also keen to discover if this feedback loop influences communication between other tissue types within our bodies, like skeletal muscle.
If so, this could explain an awful lot, as many known factors of biological aging, including stress and stress mitigators, weight, and exercise, all influence some part of this brain to fat system – another example of just how physiologically linked we are to the world around us.
This research was published in Cell Metabolism.