A widely available and inexpensive antidepressant drug may soon save lives from an altogether different kind of disease.
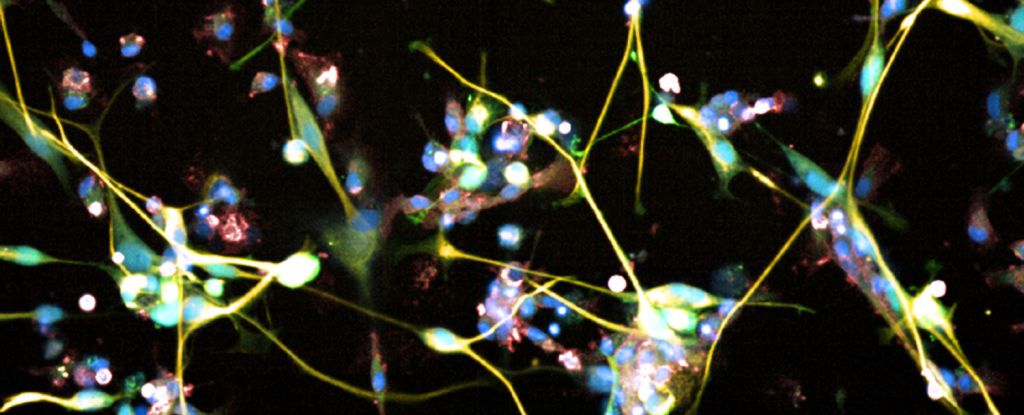
The growth of the most aggressive and deadly brain cancer, glioblastoma, was effectively suppressed in both ex vivo human tissue samples and in living mice by an FDA approved serotonin modulator currently used to treat major depression.
It’s not a cure, but it may offer some relief and constitute an effective part of a treatment regime for glioblastoma patients. Human clinical trials are the next step; patients are cautioned against self-medicating at this stage.
“The advantage of vortioxetine is that it is safe and very cost-effective,” says neurologist Michael Weller of the University Hospital Zurich in Switzerland. “As the drug has already been approved, it doesn’t have to undergo a complex approval procedure and could soon supplement the standard therapy for this deadly brain tumor.”
Glioblastoma is rare, but when it strikes, it’s hard to defeat. The tumor typically appears on the brain or brain stem, growing quickly and aggressively. There’s currently no cure, meaning it’s usually fatal, claiming around 95 percent of patients within five years. Treatment usually involves radiotherapy and chemotherapy, and sometimes surgery to try and remove as much of the tumor as is safe.
A noninvasive treatment that can complement existing interventions to improve outcomes is something doctors would love to have, but since few cancer drugs can cross the blood-brain barrier options are limited.
Led by molecular biologist Sohyon Lee of ETH Zurich, a team of researchers used cultivated human tissue grown from samples donated by glioblastoma patients undergoing treatment surgery, to see if any existing drugs might be effective at suppressing the growth of cancer cells. They focused primarily on antidepressant drugs, antipsychotics, and drugs used to treat Parkinson’s disease.
In all, they tested 132 drugs on tissue cultivated from 27 glioblastoma patients, cataloging more than 2,500 drug responses. And they found, surprisingly, that some antidepressant drugs were effective at suppressing the development of the cancer cells, including a serotonin modulator called vortioxetine.
One of vortioxetine’s actions is to activate signaling cascades, a series of reactions in a cell initiated by a stimulus. These cascades suppress cell division, which is the way cancers grow and spread.
Computer simulations revealed that the simultaneous cascade of neural cells and cancer cells was necessary to inhibit the cancer, which was why only some of the antidepressants were effective – they don’t all work quite the same way.
The next step was to test the drugs in a living, breathing system: mice with glioblastoma. Mice were transplanted with glioblastoma tumors, and then assigned a group. The control group was left untreated, while a second group of mice was treated with the SSRI antidepressant citalopram. Finally, a third group was treated with vortioxetine.
Comparisons 38 days after implantation of the tumor revealed the vortioxetine treatment group had significantly less tumor growth and invasiveness than the control and citalopram groups, which displayed similar outcomes to each other.
In a follow-up experiment, another sample of glioblastoma mice was treated with standard chemotherapy drugs. Another group was was given vortioxetine, while a third wasn’t given any other treatment.
Similarly, the vortioxetine group had increased survival rates 20-30 percent above those of the chemo-only group, not just in the short term, but long-term, too.
With such promising pre-clinical results, additional trials in living human patients could reveal whether we may already have a glioblastoma therapy ready to fast-track.
“We don’t yet know whether the drug works in humans and what dose is required to combat the tumor, which is why clinical trials are necessary,” Weller cautions. “Self-medicating would be an incalculable risk.”
Nevertheless, the drug shows more promise for the treatment of this devastating cancer than we’ve seen in a while, giving some hope to the estimated 250,000 individuals who are diagnosed with glioblastoma each year.
“We started with this terrible tumor and found existing drugs that fight against it,” says molecular biologist Berend Snijder of ETH Zurich. “We show how and why they work, and soon we’ll be able to test them on patients.”
The research has been published in Nature Medicine.