Helper cells in the brain called astrocytes could be encouraged to clear out the brain ‘trash’ associated with Alzheimer’s disease, according to new research that reveals more about how these neuron support cells work.
Previous research has shown that astrocytes go into power-cleaning mode when they encounter amyloid beta proteins, which can form clumps in the brains of people with Alzheimer’s that may play a role in the condition’s progress.
However, the same astrocytes can also do damage themselves, becoming toxic if they’re overwhelmed by amyloid beta clumps or the cleaning process is disrupted. That mix of good and bad was the starting point for this new study.
The researchers conducted RNA sequencing to examine patterns of gene expression in human astrocytes, before testing the roles of target pathways in mice with a modeled form of Alzheimer’s.
They observed clear physical changes in the astrocytes that turned them into recycling machines through a process known as autophagy, allowing them to clear out accumulations of amyloid beta proteins.
“We discovered that astrocytes possess an inducible autophagic plasticity in response to amyloid beta, and this regulatory mechanism exhibits a pivotal function in removing amyloid beta plaques,” write the researchers in their published paper.
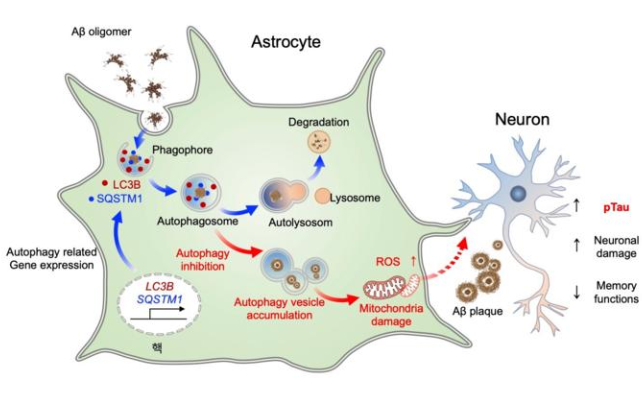
The study emphasizes the importance of autophagy in amyloid beta removal, confirming this cleaning mode can be dialed up or down with the right genetic tweaks.
When astrocytes in the brains of mice were genetically modified to trigger their autophagy forms, damage to neurons caused by Alzheimer’s-like symptoms reversed and cognitive functions and memory improved, the researchers discovered.
Two key genes involved, called SQSTM1 and LC3B, produce a protein that helps eat up unwanted material in the brain. SQSTM1 also creates a protein that tags material for destruction. These proteins have been found at higher levels in the brains of people who have died with Alzheimer’s.
While scientists remain unsure whether Alzheimer’s causes the dangerous buildup of amyloid beta and another protein called tau, or it’s the other way around, understanding the neurological mechanisms involved in their management could inform future investigations on the condition’s progress.
It also gives researchers another line of attack: most studies looking at potential Alzheimer’s treatments focus on the neurons, whereas here the team focused on the astrocyte cells that support the neurons (which are far more abundant).
We’re continuing to discover more and more about how Alzheimer’s gets started, how it progresses and damages the brain, and ways in which it might be stopped or prevented. If and when a cure eventually appears, astrocytes and their recycling mechanisms could well play some kind of role.
“The pharmacological and genetic modulation of astrocytic autophagy pathway can be considered for boosting the detoxifying machinery of astrocytes under Alzheimer’s disease stresses and may be a therapeutic strategy for ameliorating Alzheimer’s disease pathology,” write the researchers.
The research has been published in Molecular Neurodegeneration.