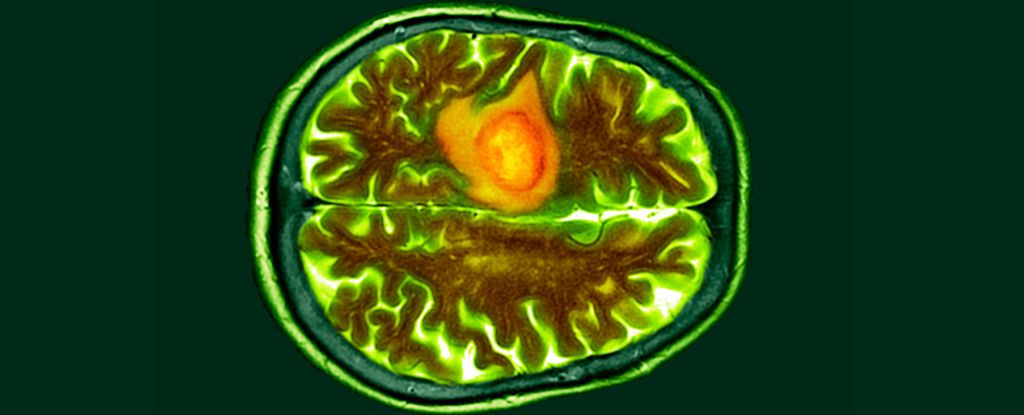
Scientists have come up with a new way to detect brain cancer that is faster and less invasive than a surgical biopsy.
Only 100 microliters of blood are needed to run this novel ‘liquid biopsy’, and within an hour, the method can detect biomarkers associated with glioblastoma – the deadliest and most common type of brain tumor.
The method surpasses all other existing tests and markers for glioblastoma with excellent accuracy. The developers of the prototype say it has “near turn-key functionality“.
The breakthrough was achieved by a US and Australian team, led by scientists from the University of Notre Dame in the US. Their proof of concept isn’t perfect, but it is an important step forward for diagnosis.
The test is based on sensing mutated blood biomarkers, called epidermal growth factor receptors (EGFRs), which are overexpressed in certain cancers, like glioblastoma.
These blood biomarkers are found tucked inside extracellular vesicles, which are small packages that contain proteins, lipids, and genetic material from their original cells.
“Extracellular vesicles or exosomes are unique nanoparticles secreted by cells,” explains biomolecular engineer Hsueh-Chia Chang from Notre Dame.
“They are big – 10 to 50 times bigger than a molecule – and they have a weak charge. Our technology was specifically designed for these nanoparticles, using their features to our advantage.”
To detect the molecules released from the cells of cancerous tumors, researchers bathed a supersensitive biochip in an untreated sample of blood plasma.
This chip costs less than US$2, and it is equipped with a tiny sensor about the size of a ball in a ballpoint pen. The crucial interface contains antibodies that are drawn to exosomes carrying mutated EGFRs.
When these EGFRs attach to the biochip, a voltage change occurs in the plasma solution, triggering a high negative charge. This is indicative of possible cancer.
In experiments, the biochip was tested on clinical blood samples from 20 patients with glioblastoma and 10 healthy individuals. One chip was used for each test.
Ultimately, the liquid biopsy detected the presence of cancer biomarkers with excellent accuracy and a very low p value, indicating the test is highly replicable.
“Our electrokinetic sensor allows us to do things other diagnostics cannot,” explains biomolecular engineer Satyajyoti Senapati from Notre Dame.
“We can directly load blood without any pretreatment to isolate the extracellular vesicles because our sensor is not affected by other particles or molecules. It shows low noise and makes ours more sensitive for disease detection than other technologies.”
In experiments, Senapati and colleagues say, the biochip can accurately detect and quantify exosome concentrations, even when they are as low as 0.01 percent.
This could have “great implications” for cancer research, biomarker discovery, and disease monitoring, the team argues – and not just for brain cancer.
But there are still some kinks to work out.
Mutated EGFRs are not just connected to glioblastomas. They are also linked to other diseases, like colorectal cancers.
“Therefore, such an EGFR active and total signature might not necessarily indicate the presence of glioblastoma specifically,” the authors write.
“Likewise, patients with glioblastoma can have amplified or mutated EGFR but can also have non-EGFR driven forms of the disease.”
This means the test isn’t able to diagnose all cases of potential glioblastoma. It also can’t say for sure what type of cancer someone has, where it is located in their body, or to what stage the disease has progressed.
To create a more specific test, the team says they need to analyze larger cohorts of glioblastoma patients to figure out what biomarkers in their blood set them apart.
“The current diagnostic platform can be scaled up for such large-library testing of untreated plasma from a large cohort of cancer patients to establish specific profiles for different cancers at different stages,” the researchers conclude.
The study was published in Communications Biology.