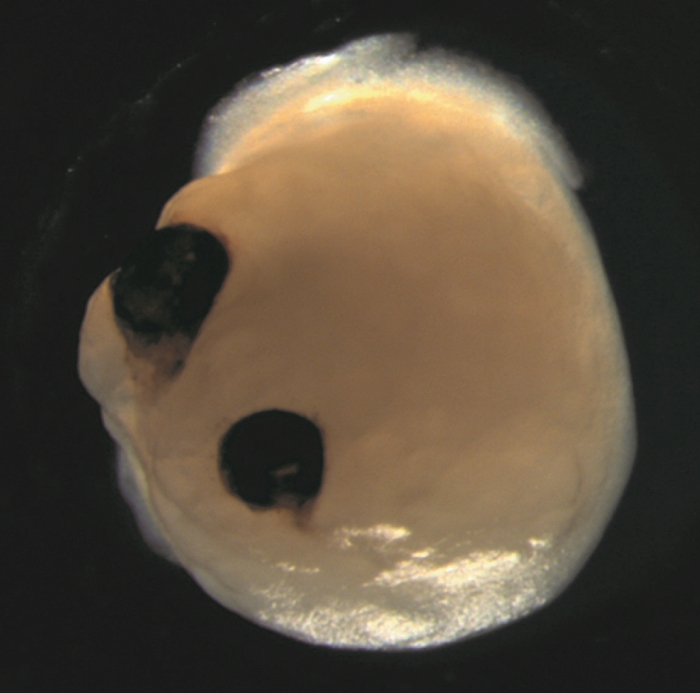
Lab-grown mini-brains stem cells spontaneously evolved rudimentary eye structures, scientists reported in an intriguing 2021 paper.
Tiny human-derived brain organoids grown in dishes grew two bilaterally symmetrical eye cups, reflecting the development of eye structures in human embryos. This incredible result could help us better understand the process of eye differentiation and development, as well as eye diseases.
“Our work underscores the remarkable ability of brain organoids to generate primitive sensory structures that are light-sensitive and harbor cell types similar to those found in the body,” said neuroscientist Jay Gopalakrishnan of the University Hospital Düsseldorf in Germany in a statement from 2021.
“These organoids can help study brain-eye interactions during embryonic development, model congenital retinal diseases, and generate patient-specific retinal cell types for personalized drug testing and transplantation therapies.”
Brain organoids are not real brains as you might think of them. They are small, three-dimensional structures grown from induced pluripotent stem cells — cells taken from adult humans and reconstructed into stem cells that have the potential to grow into many different types of tissue.
In this case, these stem cells are coaxed into growing into clumps of brain tissue without anything reminiscent of thoughts, emotions, or anything like that consciousness. Such “mini-brains” are used for research purposes where using actual living brains would be impossible or at least ethically precarious – for example, to test drug reactions or to observe cell development under certain unfavorable conditions.
This time, Gopalakrishnan and his colleagues attempted to observe eye development.
In previous research, other scientists had used embryonic stem cells to grow eyecups, the structures they develop into almost the entire eyeball during embryonic development. And other research had developed eyecup-like structures from induced pluripotent stem cells.
Rather than grow these structures directly, Gopalakrishnan’s team wanted to see if they could be grown as an integral part of brain organoids. This would add the benefit of seeing how the two tissue types can grow together rather than just growing optical structures in isolation.
“Eye development is a complex process, and understanding it could allow to underpin the molecular basis of early retinal diseases,” the researchers said wrote in their newspaper.
“Thus, it is crucial to examine visual sacs, which represent the anlage of the eye whose proximal end is attached to the forebrain, which is essential for proper eye formation.”
Previous work on developing organoids showed evidence of retinal cells, but these did not develop optical structures, so the team changed their protocols.
They did not attempt to force the development of purely neural cells in the early stages of neural differentiation and added retinol acetate to the culture medium as an aid in eye development.
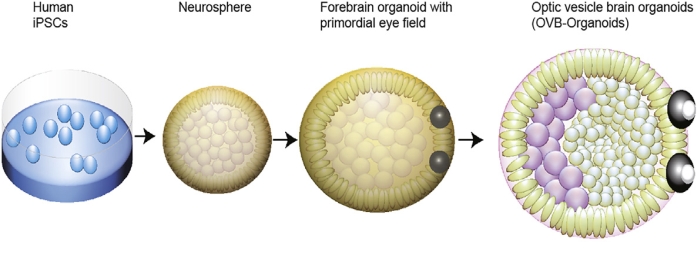
Their carefully nurtured baby brains were forming visual cups as early as day 30 of development, with the structures clearly visible by day 50. This is consistent with the Timing of eye development in the human embryomeaning these organoids could be useful for studying the intricacies of this process.
There are other implications as well. The eyecups contained different types of retinal cells organized into neural networks that responded to light, and even contained lens and corneal tissue. Finally, the structures demonstrated retinal connectivity with regions of brain tissue.
“In the mammalian brain, nerve fibers of retinal ganglion cells extend to connect to their brain targets, an aspect never before shown in an in vitro system,” said Gopalakrishnan.
And it’s reproducible. Of the 314 brain organoids the team grew, 73 percent developed eyecups. The team hopes to develop strategies to keep these structures viable for longer periods of time to conduct more in-depth research with great potential, the researchers said.
“Brain organoids containing optic vesicles that exhibit highly specialized neuronal cell types can be engineered, paving the way to generate personalized organoids and retinal pigment epithelial layers for transplantation,” they said wrote in their newspaper.
“We believe that [these] are next-generation organoids that help model retinopathies that arise from early neurodevelopmental disorders.”
The research was published in cell stem cell.
A version of this article was first published in August 2021.