A new technique that produces 3D models of individual crystals has opened a window for scientists to see the subtle deviations that emerge in their otherwise perfect patterns.
Researchers from New York University (NYU) went back to the drawing board on how to look deep inside solids made of repeating units, and determine how they grow.
With a short wavelength roughly the same size as many of the repeating units that make up crystals, X-rays have long allowed scientists to infer how a crystal’s components fit together by measuring the angle at which the rays are diffracted.
For all its ingenuity, though, X-ray crystallography has its limits, which are summed up rather neatly by the opening sentence of a new paper published in Nature Materials this month: “Structures of molecular crystals are identified using scattering techniques because we cannot see inside them.”
The paper describes a new technique that promises to finally change that fact – albeit not for crystals composed of repeating units of individual atoms.
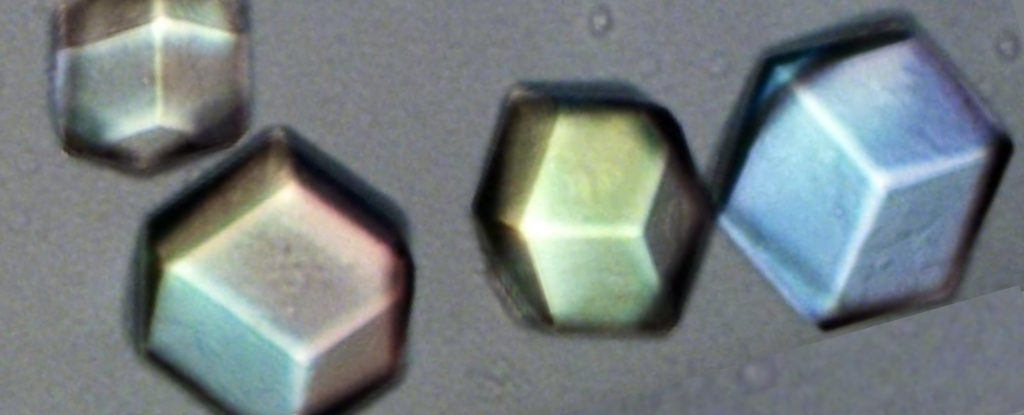
Instead, it concerns crystals composed of patterns based on colloidal particles, which are large enough to be seen under a conventional microscope and to manipulate in a manner that would be impossible for atoms.
Studying such crystals has allowed for advances in the understanding of crystal dynamics. The researchers cite experiments with colloidal structures that shed light on the formation and evolution of dislocations within crystal structures.
Like X-ray crystallography, this technique has limits. Difficulties in finding reliable ways to image relatively complex colloidal crystals have meant their study has so far largely been limited to thin, simple structures formed from a single constituent particle.
Many atomic-scale crystals, by contrast, are made up of two or more elements and form complex, three-dimensional structures.
The new technique pioneered by the NYU team promises to allow study of colloidal analogues of these relatively complex lattices. The technique builds on some of the team’s previous work, in which they developed a process called “polymer-attenuated Coulombic self-assembly”, or PACS.
PACS uses individual colloidal particles’ electric charges to draw them into crystal lattices, allowing for the reliable construction of binary colloidal crystals – crystals formed by molecules composed of two different species of particle in the same way that, say, crystals of table salt are formed from sodium and chlorine.
The new study demonstrates the effectiveness of seeding these individual colloidal particles with a fluorescent dye to distinguish one species from the other – and, crucially, continues to do so once they have formed crystals. This means that at long last, scientists can “look inside” a fully formed crystal and make direct observations of its innards.
As the researchers report, “We are able to distinguish all of the particles within a binary ionic crystal, and reconstruct the full internal 3D structure up to depths of ~200 layers.”
The NYU team reports several new findings they’ve already gleaned from observations.
The process known as “twinning”, where two crystals’ lattices align in such a way that they share components parts along a common plane, has long been of interest to scientists.
The researchers describe creating colloidal crystals that reproduce the atomic-scale cubic structures of several different minerals: the aforementioned alternating lattice of sodium and chlorine that forms table salt; cesium chloride, where eight chlorine atoms form a “cage” around a single cesium atom; and the somewhat more exotic example of auricupride, a compound of copper and gold, where each face of a cubic lattice of gold atoms is punctuated with a single copper atom, like a die where each and every face is a one.
In each case, the team was able to make direct observations of the evolution of twinned crystals, thus providing a direct experimental observation of how such structures arise.
“This direct observation unambiguously unravels the internal intricacies of the crystal structure, elucidating the relationship between the particle interactions and the macroscopic crystal form, including the emergence and impact of defects and twinning,” the researchers report.
The group are looking forward to the unraveling of crystals’ mysteries, more than 100 years after the discovery of X-rays allowed humanity its first hint of the intricacies of crystalline structure.
The research has been published in Nature Materials.