White could be the new beige when it comes to fat cells, following the discovery of a switch that maintains the functions of adipose tissue in mice, transforming it from a lipid-locker into a calorie-burner.
Physician scientist Brian Feldman and molecular biologist Liang Li from the University of California, San Francisco carried out a number of experiments on human cell cultures and mice engineered with a switch for a gene they hypothesized regulates the maintenance of our fat.
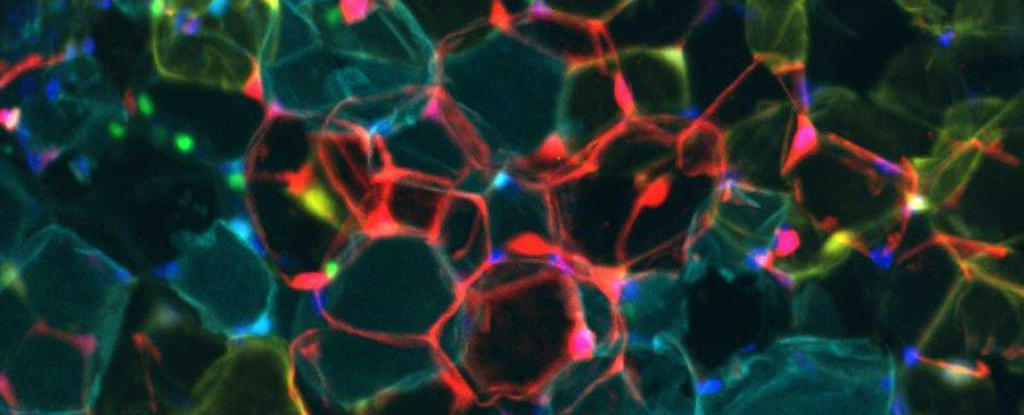
By depriving the mice of the transcription factor Klf15, the researchers were able to transform the identity of ‘deep storage’ white adipose tissue (WAT) into a more transient, thermoregulating form called brown adipose tissue (BAT).
Fat tissues typically come in two varieties in mammals. White fat is like a long-term savings deposit account for calories, locking lipids away beneath our skin and around our soft internal organs to serve as shock absorbers and insulators.
Brown fat, on the other hand, is darkened by a generous number of cellular power-generators primed to burn through its fuel supply at a moment’s notice. Scarce in adult humans, babies (and hibernating mammals) are blessed with significant amounts of BAT to keep their sleeping bodies toasty warm.
For most of our evolutionary history this age-relative balance of WAT versus BAT has served us well. Mature members of our species keep warm by using fat as a fuel for movement, while immobile newborns benefit from a more passive form of temperature regulation.
Of course, in environments where dietary fats are abundant and mobility is limited, it’s far too easy to invest a bounty of unused lipids in white fat storage, often to the increasing detriment of our health.
Nature hasn’t made it easy to retrieve those fats once they’re stored, either, inspiring researchers to search for ways to switch fat tissue types.
“For most of us, white fat is not rare and we’re happy to part with some of it,” says Feldman.
With Feldman’s earlier investigations hinting at a role for Klf15 in fat metabolism he decided to dig deeper and pin down its specific functions.
The first big clue came in analyses comparing the quantities of Klf15 between the different kinds of fat tissue. The transcription factor was relatively abundant in the white cells, prompting Feldman and Li to ask what might happen if they deprived the tissues of the protein.
Knowing isoproterenol stimulates brown tissue into generating heat, the pair administered doses of the compound into human WAT cultures and wild-type mice. Signs were clear that there was a relationship between the activation of brown fat and levels of Klf15, with follow-up investigation revealing an adrenergic receptor called Adrb1 was the critical link.
A related receptor called Adrb3 was already known to researchers, with animal studies raising hopes that stimulating it could encourage white fat cells to change identity and become more brown-like, making it a little easier to shed their stores.
Clinical trials are exploring whether Adrb3 agonists improve metabolic health in humans, though based on findings that the receptor isn’t detectable in human WAT, Feldman is optimistic that Adrb1 could serve as a more suitable therapeutic target.
In an ultimate test, mice engineered with a kind of Klf15-gene toggle were found to increase their expression of Adrb1, making white adipose tissue more ‘beige’.
Finding a way to generate a similar reaction in humans using pharmaceuticals could help overcome the hurdles many face in breaking into their fat reserves, without the side effects that come with many other approaches.
“A lot of people thought this wasn’t feasible,” says Feldman.
“We showed not only that this approach works to turn these white fat cells into beige ones, but also that the bar to doing so isn’t as high as we’d thought.”
This research was published in the Journal of Clinical Investigation.