For the first time, physicists have succeeded in creating a strange, fragile kind of structure in a laboratory known as a trilobite Rydberg molecule.
Building and observing these exotic atomic structures has given scientists new insights into the quantum activity of electrons as they scatter near atoms.
Since their chemical bonds are unlike any other (that we know), the findings open avenues for developing better theoretical models of molecules, and understanding their dynamics.
Rydberg molecules are created from a type of atom known as a Rydberg atom. In a normal atom, you have the nucleus, surrounded by its tiny swarm of electrons. If you add just a bit of energy to the atom, the electron swarm puffs out a little, making the atom just that teensy bit bigger and looser.
A Rydberg atom is what you get when you add a lot of energy under conditions that allow it to still hold onto its electrons. It puffs up quite large, for an atom, many microns across, and the electrons are about as loosely bound as they can get without flying off.
Because they’re so loosey-goosey, Rydberg atoms sort of behave in an exaggerated way, which makes them useful for conducting experiments.
Molecules are arrangements of atoms that glom together in some way, such as by co-parenting electrons or perhaps through contrasting charges. If you use a Rydberg atom, you get a Rydberg molecule, but the way the atoms stick to each other can be very different from the bonds that join more conventional molecules.
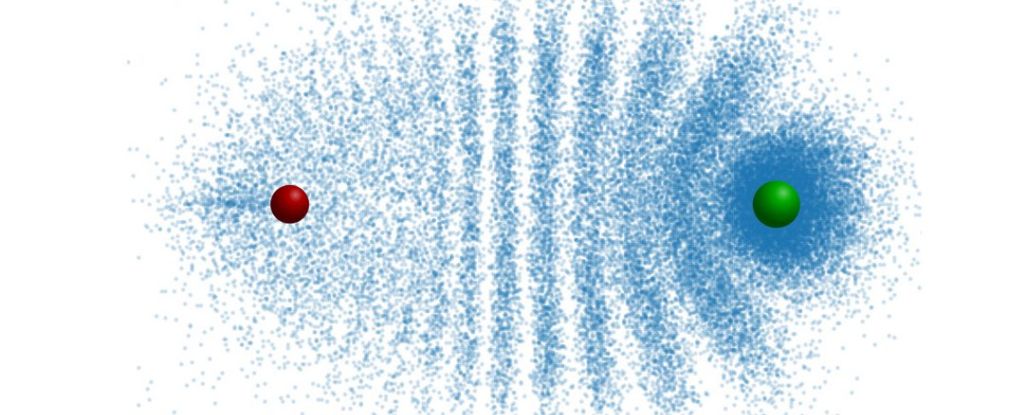
And they can look very different, with distribution electron patterns that can resemble, say, a trilobite, or a butterfly.
Led by physicist Max Althön of the University of Kaiserslautern-Landau, a team of scientists in the laboratory of Herwig Ott has created, for the first time, pure trilobite Rydberg molecules.
They started with atoms of rubidium, ultra-cooled to just 0.0001 degrees above absolute zero. Then, they used a laser to excite some of the atoms into Rydberg states.
“In this process, the outermost electron in each case is brought into far-away orbits around the atomic body,” Ott says. “The orbital radius of the electron can be more than one micrometer, making the electron cloud larger than a small bacterium.”
A Rydberg molecule can be created by bringing a ground state atom – one that has not been excited into a Rydberg state – into the puffy electron swarm of the Rydberg atom, the two atoms sticking together not with standard chemical bonds, but a strange quantum attraction.
“It is the quantum mechanical scattering of the Rydberg electron from the ground state atom, which sticks the two together,” Althön explains.
“Imagine the electron rapidly orbiting around the nucleus. On each round trip, it collides with the ground state atom. In contrast to our intuition, quantum mechanics teaches us that these collisions lead to an effective attraction between the electron and the ground state atom.”
Because of the repeated collisions, the electrons become distributed into an interference pattern that resembles the segmented carapace of a trilobite.
It has some other fascinating and strange properties, too. The length of the molecular bond is almost the same size as the Rydberg orbit, which is to say, quite large for atomic scales. And the strength of the attraction between the electron and the ground state atom is quite high, too.
This means that Rydberg molecules have a higher electric dipole moment than any other molecule; that is, the separation between positive and negative electric charges, also known as polarity.
The trilobite Rydberg molecules observed by Althön and his colleagues have an electric dipole moment of more than 1,700 debye, and that is extremely high. For water molecules, this measure is less than 2 debye.
The ability not just to create, but probe pure trilobite Rydberg molecules gives physicists a new tool for testing and understanding the quantum realm.
It also has potential applications for quantum information processing. And, the researchers say, it could be more broadly applied to study these strange molecules across different species.
“In conclusion, we have measured two vibrational series of pure trilobite Rydberg molecules by employing three-photon photoassociation,” they write. “With this method, the creation of trilobite molecules in any element that has a negative s-wave scattering length should be possible.”
The research has been published in Nature Communications.