The sex drives of male mice seem to arise from a single, newly identified brain circuit, a new study reveals.
This circuit governs a male mouse’s sex drive and its resulting behavior and reward experience, suggesting it plays a vital role in compelling them to reproduce.
And while that’s interesting, the US researchers are curious about more than just motivations for mouse sex. This brain circuitry is probably ancient, they note, given its apparent importance in persuading male mice to mate.
A brain circuit this fundamental is unlikely to change much during the course of evolution, the team says, so there is reason to suspect it’s relatively standard, at least among male mammalian brains.
“We’ve singled out a circuit in male mammals’ brains that controls sexual recognition, libido, and mating behavior and pleasure,” says senior author Nirao Shah, a neurobiologist at Stanford University.
Studying this circuit could yield new insights about mammals overall, Shah and colleagues say, and they’re endeavoring to research equivalent circuits in female mammals.
Since we’re mammals, those insights could also inspire new pharmaceutical drugs for humans or shed light on factors shaping human sexuality.
To identify this brain circuit, the researchers studied adult male mice whose behavior and brain activity were minimally altered by social influences. They were not only virgins but hadn’t even seen a female mouse since weaning.
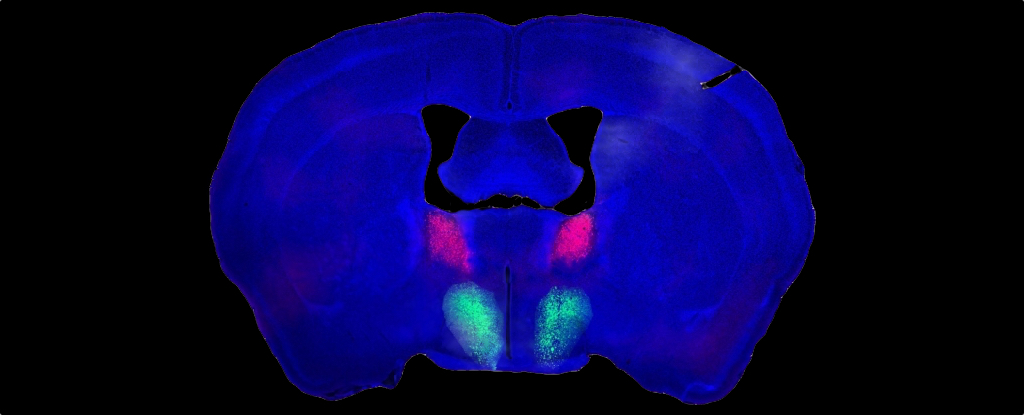
In previous research, Shah and team were intrigued by neurons extending from part of the amygdala called the bed nucleus of the stria terminalis (BNST) and linking to the preoptic area in the hypothalamus.
By manipulating those neurons, the researchers had discovered they could toggle on and off a male mouse’s ability to recognize the sex of unfamiliar mice. The new study expands on their findings, zeroing in on the neurons.
“We wanted to know exactly which of these neurons were talking to exactly which neurons in the preoptic hypothalamus once that recognition occurred,” Shah says.
One group of genetically distinct BNST neurons produces a peptide known as Substance P, the researchers report, while another unique neuron group from the preoptic hypothalamus has Substance P receptors.
Substance P-producing BNST neurons formed connections with their receptive counterparts from the preoptic hypothalamus, and they found at least one purpose those links might serve.
When they used lasers to stimulate the specific BNST neurons in a male mouse’s brain, corresponding neurons in the preoptic hypothalamus leapt into action, showing elevated activity for about 90 seconds.
Then, 10 to 15 minutes later, the mice would reliably launch into the full sequence of mating behaviors.
Substance P seems to be key, flowing from the BNST to gradually stimulate neurons in the preoptic hypothalamus and increase their activity. When the team tinkered with this process, the changes in mouse behavior were striking.
Infusing Substance P into the relevant area of a male mouse’s brain, for example, significantly boosted its ability to mate with a willing female.
And when the researchers directly activated preoptic hypothalamus neurons that have Substance P receptors, some male mice even tried to mate with inanimate objects.
Substance P also led male mice to disregard their refractory period, the variable time span after mating when male mammals have reduced interest or capacity for mating again.
The mice in this study typically have a five-day refractory period, but stimulating specific neurons in their preoptic hypothalamus led male mice to jump right back into mating even immediately after finishing.
“It took one second or less for them to resume sexual activity,” Shah says. “That’s a more than 400,000-fold reduction in the refractory period.”
Suppressing the same neurons led male mice to become celibate, Shah adds, but they seemed otherwise unaffected.
Downstream from neurons with Substance P receptors are other brain areas important for voluntary movement and pleasure, the researchers note, hinting at how all this might work – and not necessarily just in mice.
“It’s very likely there are similar sets of neurons in the human hypothalamus that regulate sexual reward, behavior and gratification,” Shah says. “And they’re probably quite similar to the ones we’ve observed in mice.”
Among other possible applications, these findings could aid development of drugs to help men manage a hyperactive sex drive or a lack of libido, the researchers say.
“If these centers exist in humans – and now we know where to look – it should be possible to design small molecules that can be used to regulate these circuits,” Shah says.
The study was published in Cell.